Patients Use Cases
A pragmatic risk stratification approach with LIVERFASt™
- Case 1
- Case 2
- Case 3
- Patient 1 | 66 Male
Clinical Information
- Presents to PCP with moderate fatigue and malaise
- Patient gained 20 pounds over the last 12 months
- Uncontrolled Diabetic
- Currently taking Diovan HCT 320mg/25mg and Janumet 50/500mg
- Past medical history of hypertension, diabetes, and
- CKD stage 3
- No family history of medical problems
- BMI 36
- BP 160/90
- How it works
Steps
- Clinician orders LIVERFASt™ for the patient
- LIVERFASt Proprietary CPT Code 0166U
- The patient has a simple fasting blood test of the 10 biomarkers
- The lab provides results of the 10 biomarker results
- The 10 biomarker results are input into Fibronostics web portal
- Fibronostics’ AI technology generates LIVERFASt™ results immediately
- Before LIVERFASt™
Laboratory Results
- Fasting Blood Glucose: 260 mg/dl
- Triglycerides: 165 mg/dl
- HDL: 50 mg/dl
- LDL: 140 mg/dl
- AST: 65 IU/L
- ALT: 70 IU/L
Clinical Assessment
- Poorly controlled Diabetes Mellitus
- Morbid Obesity
- Hypertension
- Elevated Liver Function Tests
What to do next?
- Risk management of Diabetes Mellitus
- Hypertension management
Order LIVERFASt™
“There should be a high index of suspicion for NAFLD and NASH in patients with type 2 diabetes” (Chalassani N et al. Hepatology 2018. AASLD CPG)
“In patients with type 2 diabetes, the presence of NAFLD should be looked for irrespective of liver enzyme levels, since type 2 diabetes patients are at high risk of disease progression “ (Chalassani N et al. Hepatology 2018. AASLD CPG)
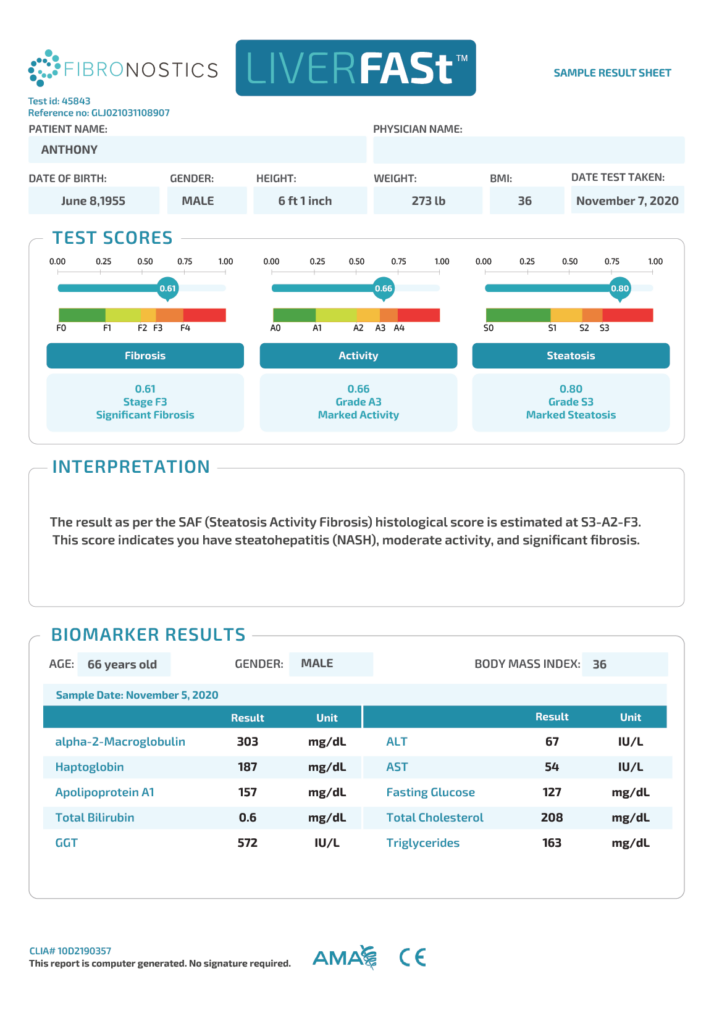
- LIVERFASt™ Results
Fibrosis
- 0.61
- F3
- Significant Fibrosis
Activity
- 0.66
- A3
- Marked Activity
Steatosis
- 0.80
- S3
- Marked Steatosis
- After LIVERFASt™
Patient stratified as high risk of NASH or advanced fibrosis.
“Patients with steatosis identified by steatosis biomarkers and having metabolic medium/high risk (indicative of significant fibrosis or cirrhosis using fibrosis biomarkers), in the presence or not of abnormal liver enzymes should be referred to a specialist for in-depth assessment of disease severity, decision to perform liver biopsy, initiate monitoring/therapy.” (EASL-EASD-EASO. J Hepatol 2016 CPG)
- Patient 1 | 66 Male
Clinical Information
- Presents to PCP with moderate fatigue and malaise
- Patient gained 20 pounds over the last 12 months
- Uncontrolled Diabetic
- Currently taking Diovan HCT 320mg/25mg and Janumet 50/500mg
- Past medical history of hypertension, diabetes, and
- CKD stage 3
- No family history of medical problems
- BMI 36
- BP 160/90
- How it works
Steps
- Clinician orders LIVERFASt™ for the patient
- LIVERFASt Proprietary CPT Code 0166U
- The patient has a simple fasting blood test of the 10 biomarkers
- The lab provides results of the 10 biomarker results
- The 10 biomarker results are input into Fibronostics web portal
- Fibronostics’ AI technology generates LIVERFASt™ results immediately

- Before LIVERFASt™
Laboratory Results
- Fasting Blood Glucose: 260 mg/dl
- Triglycerides: 165 mg/dl
- HDL: 50 mg/dl
- LDL: 140 mg/dl
- AST: 65 IU/L
- ALT: 70 IU/L
Clinical Assessment
- Poorly controlled Diabetes Mellitus
- Morbid Obesity
- Hypertension
- Elevated Liver Function Tests
What to do next?
- Risk management of Diabetes Mellitus
- Hypertension management
Order LIVERFASt™
“There should be a high index of suspicion for NAFLD and NASH in patients with type 2 diabetes” (Chalassani N et al. Hepatology 2018. AASLD CPG)
“In patients with type 2 diabetes, the presence of NAFLD should be looked for irrespective of liver enzyme levels, since type 2 diabetes patients are at high risk of disease progression “ (Chalassani N et al. Hepatology 2018. AASLD CPG)
- LIVERFASt™ Results
Fibrosis
- 0.61
- F3
- Significant Fibrosis
Activity
- 0.66
- A3
- Marked Activity
Steatosis
- 0.80
- S3
- Marked Steatosis
- After LIVERFASt™
Patient stratified as high risk of NASH or advanced fibrosis.
“Patients with steatosis identified by steatosis biomarkers and having metabolic medium/high risk (indicative of significant fibrosis or cirrhosis using fibrosis biomarkers), in the presence or not of abnormal liver enzymes should be referred to a specialist for in-depth assessment of disease severity, decision to perform liver biopsy, initiate monitoring/therapy.” (EASL-EASD-EASO. J Hepatol 2016 CPG)
- Patient 1 | 66 Male
Clinical Information
- Presents to PCP with moderate fatigue and malaise
- Patient gained 20 pounds over the last 12 months
- Uncontrolled Diabetic
- Currently taking Diovan HCT 320mg/25mg and Janumet 50/500mg
- Past medical history of hypertension, diabetes, and
- CKD stage 3
- No family history of medical problems
- BMI 36
- BP 160/90
- How it works
Steps
- Clinician orders LIVERFASt™ for the patient
- LIVERFASt Proprietary CPT Code 0166U
- The patient has a simple fasting blood test of the 10 biomarkers
- The lab provides results of the 10 biomarker results
- The 10 biomarker results are input into Fibronostics web portal
- Fibronostics’ AI technology generates LIVERFASt™ results immediately

- Before LIVERFASt™
Laboratory Results
- Fasting Blood Glucose: 260 mg/dl
- Triglycerides: 165 mg/dl
- HDL: 50 mg/dl
- LDL: 140 mg/dl
- AST: 65 IU/L
- ALT: 70 IU/L
Clinical Assessment
- Poorly controlled Diabetes Mellitus
- Morbid Obesity
- Hypertension
- Elevated Liver Function Tests
What to do next?
- Risk management of Diabetes Mellitus
- Hypertension management
Order LIVERFASt™
“There should be a high index of suspicion for NAFLD and NASH in patients with type 2 diabetes” (Chalassani N et al. Hepatology 2018. AASLD CPG)
“In patients with type 2 diabetes, the presence of NAFLD should be looked for irrespective of liver enzyme levels, since type 2 diabetes patients are at high risk of disease progression “ (Chalassani N et al. Hepatology 2018. AASLD CPG)
- LIVERFASt™ Results
Fibrosis
- 0.61
- F3
- Significant Fibrosis
Activity
- 0.66
- A3
- Marked Activity
Steatosis
- 0.80
- S3
- Marked Steatosis
- After LIVERFASt™
Patient stratified as high risk of NASH or advanced fibrosis.
“Patients with steatosis identified by steatosis biomarkers and having metabolic medium/high risk (indicative of significant fibrosis or cirrhosis using fibrosis biomarkers), in the presence or not of abnormal liver enzymes should be referred to a specialist for in-depth assessment of disease severity, decision to perform liver biopsy, initiate monitoring/therapy.” (EASL-EASD-EASO. J Hepatol 2016 CPG)





