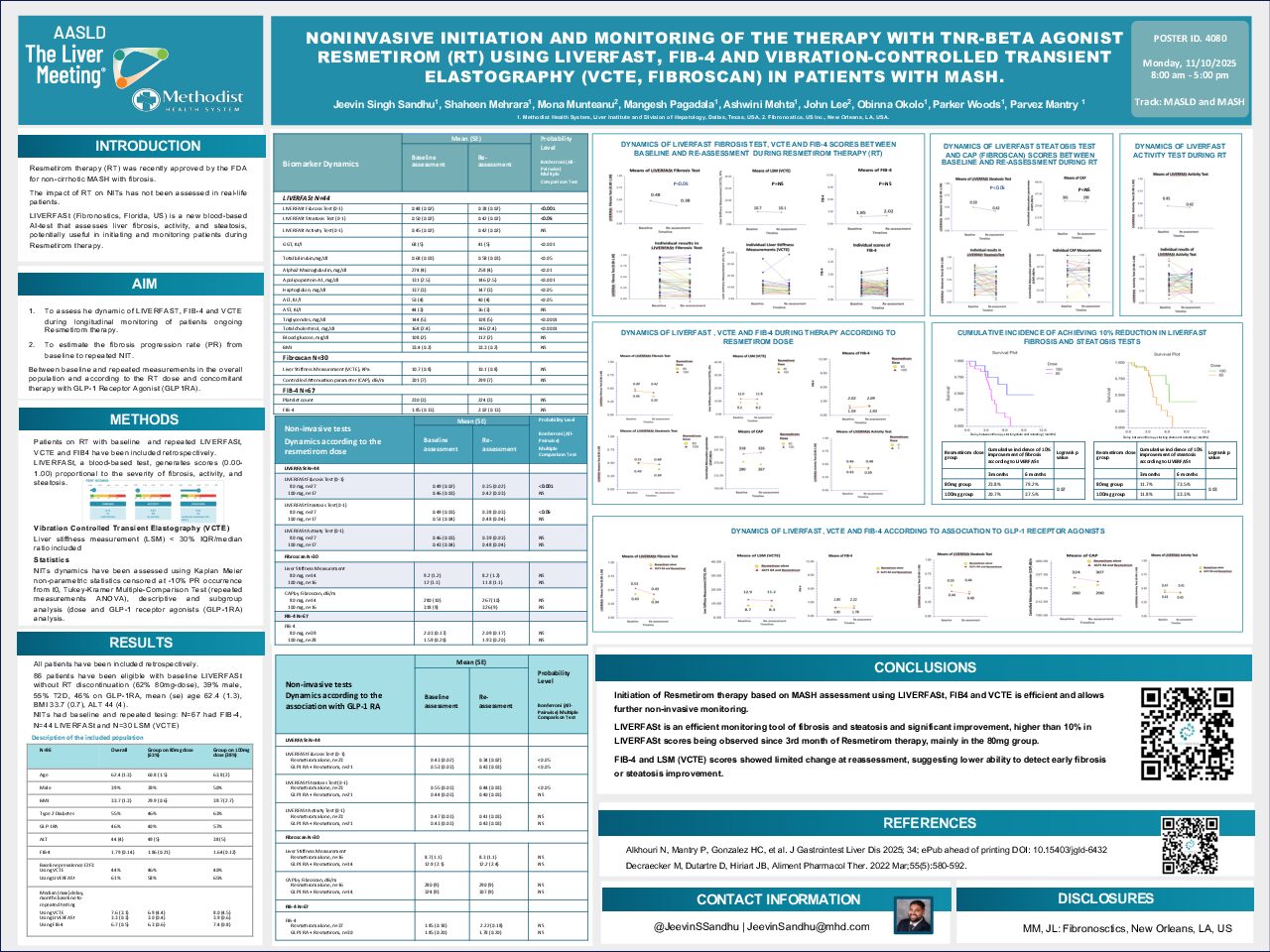
The poster presents a retrospective study on the use of non-invasive tests (NITs) to initiate and monitor Resmetirom (RT) therapy in patients with non-cirrhotic MASH (Metabolic dysfunction-associated steatohepatitis) with fibrosis, a treatment recently approved by the FDA.
Key Aims
- To assess the dynamic changes in LIVERFASt, FIB-4, and VCTE scores during longitudinal monitoring of patients on RT.
- To estimate the fibrosis progression rate (PR) from baseline to repeated NIT measurements, considering the RT dose and concomitant use of GLP-1 Receptor Agonists (GLP-1RA)